Text editing properties
In the case of chemical documents it is necessary to distinguish between two kinds of texts:
structural text (atomic symbols, functional groups, atom labels) and
other text (captions, reaction conditions, annotations etc.). The structural text strings are connected to the atoms, whereas the latter can be placed freely, usually into text boxes and can be edited independently. Of course, in these chemical applications only a minimal amount of text editing features can be found, like the basic manipulation of fonts, styles, sizes and colours. The technique is mostly that of used in other Windows applications.
The editing of structural text is the least convenient and limited in Accelrys Draw (the old, but more flexible ISIS/Draw mode can optionally be activated) and MarvinSketch (obviously only database applications have been taken into consideration). In certain cases, the method used in ChemDoodle is also complicated (e.g. the adding of Greek letters). If editing of a special atom label is difficult, the solution is simply to create a caption text and after positioning to group it with the parent structure.
Apart from ChemDoodle, the parameters of structural and caption texts are not used globally. There are five independent settings in DrawIt, three in Accelrys Draw and two in the others. The caption text editing properties are the best in ChemSketch and ChemBioDraw.
While special chemical symbols are available from the programs, the last versions of ChemBioDraw and ChemDoodle have a full floating character map for quick selecting and pasting of any characters of the installed fonts.
The creation of textual tables is supported in ChemBioDraw with the aid of the Tab key, but a complete advanced table editor supporting text & structures is also available here. Similarly, the latest ChemSketch also possesses an excellent table editor. DrawIt contains also a good table editor, but for captions only. Apart from the automatic alignment tools in Accelrys Draw, ChemDoodle, MarvinSketch and Chemistry 4-D Draw, no special help is available for table editing.
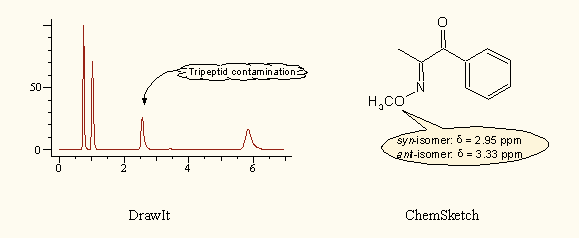
DrawIt and ChemSketch support the direct creation of framed annotations (callouts), which can be very useful in case of spectra or chromatograms. Although these can be manually assembled in the other programs, too, here they are ready to use and automatically resize when editing their content:
 International characters
International characters – These programs are widely used all over the world, therefore the support of national characters should be flawless. Unfortunately, 20 years after the rise of MS Windows not all of these program applications are faultless. The test was made using Hungarian accented and Islandic characters:
- Accelrys Draw 4.2 – no problems
- ChemBioDraw – version 11-12: incomplete support; version 13-14: only Arial, Times New Roman and Courier fonts are supported
- ChemDoodle – no problems, full unicode suppoert
- ChemSketch 2015 – incomplete support
- Chemistry 4-D Draw – no problems
- DrawIt 9 – incomplete support (foreign characters look OK in ReportIt, but some of them disappear on pasting into other applications)
- MarvinSketch – no problems
As the display and keyboard input of foreign characters are depending on the OS version, language and setup, the above results cannot be generalised.
Templates and group abbreviations
Apart from Chemistry 4-D Draw, these applications contain several ready chemical template pages of different ring systems, amino acids, carbohydrates, etc. and these can be pasted quickly into the document. The programs allow the user to create custom templates. Of course, the technique of the creation and use of the templates are different in each program; perhaps the solution and organisation used by ChemSketch is the most advanced one. A large amount of additional chemical, biochemical, biological, manufacturing, etc. templates for ChemBioDraw are available as downloadable support from the CambridgeSoft's site.
The so-called abbreviated groups (labels, superatoms, nicknames) are shorthand text string representations of functional groups (COOtBu, NO
2), meanwhile they keep their chemical significance with the underlying structure hidden – a highly important feature in the case of databases. There are several methods to add such linear structural formulas (side-chains) with or without chemical significance:
-
You draw the full structure and contract a substituent to a chemically meaningful text label, if necessary. The process can be reversed, the textual abbreviation can be expanded to structure. This feature is relevant mainly when storing molecules in databases.
- Accelrys Draw: supported
- ChemBioDraw: supported
- Chemistry 4-D Draw: works only in the case of a few predefined simple substituents
- ChemDoodle: supported (with some formatting problems)
- ChemSketch: not supported
- DrawIt: imperfect even in the case of very simple side-chains or groups. After abbreviation, the side-chain often cannot be expanded.
- MarvinSketch: supported
- Important, commonly used or user defined substituents are stored and can be added by a few keystrokes as atom labels or expanded structures. This method is available in Accelrys Draw, ChemBioDraw, ChemDoodle and MarvinSketch. Other programs support it with limitations.
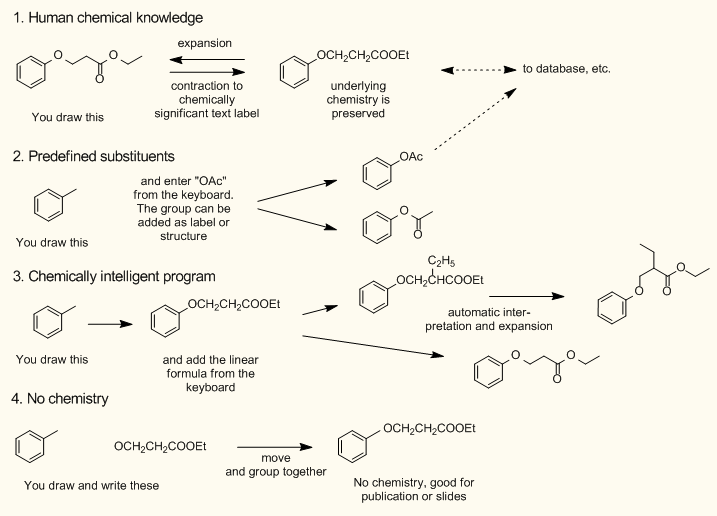
- You add the substituent or side-chain in linear textual form and the parser of the program interprets and expands it. The expression may contain parentheses, too (ChemBioDraw, DrawIt and ChemSketch). The algorithm of ChemBioDraw seems to be the most advanced one, you can even add substituents to the linear formula. Previously defined abbreviations may have two connection points. The expansion is a fully automated process and the outlook is not always according to the user's taste.
- Accelrys Draw: not supported.
- ChemBioDraw: any text label can be added, even complex and branched ones are interpreted and expanded.
- Chemistry 4-D Draw: any textual linear form can be added, but only the very basic ones can be expanded.
- ChemSketch: any textual linear form can be added and even complex ones are interpreted and expanded.
- ChemDoodle: any textual linear form can be added, even complex and branched ones are interpreted and expanded. Sometimes bonds have to be added to help the algorythm: -CH2-CH(CH2-NH2)-OH
- DrawIt: any textual linear form can be added, but only the very basic ones can be expanded.
- MarvinSketch: not supported.
Sprouting of bonds from the "letters" of the side-chain is possible in ChemBioDraw and DrawIt. While in DrawIt they are pure graphics only, ChemBioDraw interprets them on-the-fly chemically.
- The crude method: when chemistry is unimportant, you can simply create a text string and group it to the structure. Apart from MarvinSketch, it can be done in every program.
Summary: These methods are the most advanced in ChemBioDraw and ChemSketch in the second place. If the underlying chemistry is unimportant, all programs are similar. It is difficult to decide which of the above methods is the better one for the addition of substituents: predefined structures or on-the-fly interpretation of linear formulas. Each of them has its own advantages and weak points.
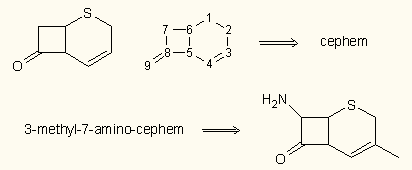
ChemBioDraw, ChemDodle and DrawIt allow the use of stacked labels (multiple lines).
Chemistry 4-D Draw provides its own solution. The Pro version also has an intelligent chemical nomenclature module. Therefore it contains only a few built-in templates (the most important rings and substituents). In addition, every molecule can be saved as a fragment (like the cephem ring in the right figure) that can later be recalled by its name, even in substituted form. A similar mechanism exists in Accelrys Draw, too. The structure resolver includes a feature that allows the user's internal database to be updated with the name/structure pairs.
Chemical intelligence, nomenclature
As it has already been discussed, several programs are capable of parsing the linearised forms of substituents and convert them into structures. All recent programs are able to assign systematic names to structures according to IUPAC rules, and
vice versa. Therefore, these are very convenient when you are generating chemical names.
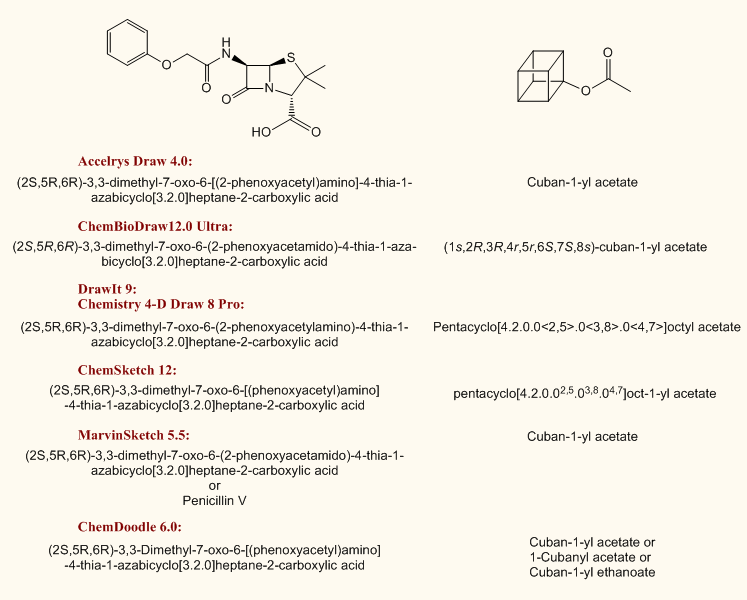
These modules are very intelligent and are difficult to cheat, although not faultless, especially on slippery fields of nomenclature, such as carbohydrates. Some of their databases can be customised by adding trivial names. There are a few restrictions, anyway, for example multicomponent ionic structures will not be interpreted in DrawIt or Chemistry 4-D Draw while this is not a problem inside ChemSketch Name or ChemBioDraw. The presence of these usually extra features are depending on the purchased version or edition of the applications (see the next table). Older Accelrys Draw versions need ACD Labs Name add-in. The basic ChemSketch contains a limited structure-to-name feature only, the opposite is to be purchased separately. ChemBioDraw Ultra also possesses an appropriate module. In the latest ChemBioDraw, ChemDoodle and MarvinSketch a praiseworthy auto-update feature renames the structure on-the-fly as you modify it. The structure-to-name module of ChemDoodle is highly customizable by toggling the use of per/bis nomenclature, Hantsch-Widman system, rings over chains, ortho/meta/para and many more options. A new nomenclature module was introduced with ChemBioDraw 12 capable of dealing with more complicated polycyclic condensed ring systems. Basic DrawIt contains only demo capabilities. If you need more, purchase the ACD/Name application, which wins in a canter even in the jungle of oligosaccharides. (
Example)
Summary of IUPAC modules in the different versions of the applications
| Program: |
Structure to name |
Name to structure |
|
Accelrys Draw 4.2, free academic
and commercial versions |
yes |
yes |
|
| ChemBioDraw 14 |
Ultra version only |
Ultra version only |
|
ChemSketch 2015, freeware
and commercial versions |
up to 50 atoms/3 rings |
no |
|
Chemistry 4D Draw 8, Standard
Pro |
yes
yes |
no
yes |
|
| ChemDoodle 8.0 |
yes (customizable) |
yes |
|
DrawIt 9, free academic version
commercial version
|
up to 10 heavy atoms
yes
|
up to 10 heavy atoms
yes
(often odd stereochemistry) |
|
| MarvinSketch 14 |
yes |
yes |
Of the program applications in Accelrys Draw and Chemistry 4-D Draw is possible to define and add full structures with trivial names to the internal database, as already mentioned above. The names and structures then become available in name-to-structure and structure-to-name conversions.
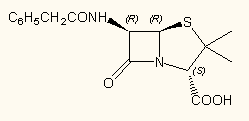
In addition, all of the programs including MarvinSketch are able to assign the stereochemistry to all asymmetric tetrahedral atoms and double bonds according to Cahn-Ingold-Prelog (CIP) priority rules. All but ChemSketch do this on-the-fly while editing the structure. The method used in ChemSketch is quite clumsy: when editing the structure, you have to delete manully the previous R and S markers and ask for re-assignement of stereochemistry. (Direct internet search from the free version can partly compensate for this benefit.)
The commercial version of ChemSketch includes an integrated dictionary of over 30 000 structures with 165 000 trivial and trade names, which can be searched by name, structure and a series of abbreviations, drug code numbers, therapeutic categories or inhibited enzymes.
As a whole, the structure-to-name service is a very useful feature for every organic chemist; the name-to-structure conversion is usually less practical: long and complicated names are difficult to enter without typo or syntax error (they can be copy/pasted from different sources, though) while short names are usually quicker to enter immediately as graphics.
Spectra and chromatograms
Some novelties in these programs are the spectral data publishing features, spectrum interpretation and database management tools. UV, IR, NMR, MS spectra and chromatograms can be imported and embedded into the documents and join with structures etc. The most versatile are DrawIt and ChemSketch (only when purchasing the appropriate add-ons). ChemBioDraw supports JCAMP and Galactic files, ChemDoodle and MarvinSketch JCAMP files. The curves can be resized, zoomed, scaled, corrected, annotated, and peak list and tables are easy to create. Finally, they can be embedded into the final document. Basic spectrum manipulations, such as Fourier transformation of the FID signals are not possible. All of the these applications enable the user to assign structures to spectra. Before making any decision, it is
highly recommended to check that what spectrum and database formats are supported by the current versions.
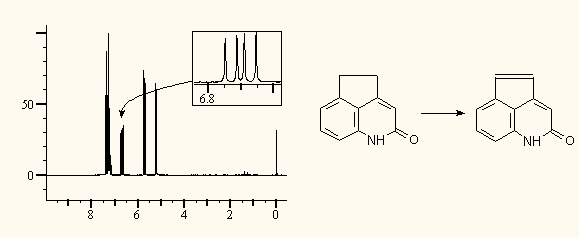
Any spectra from any related ACD/Labs application can be put in ChemSketch:
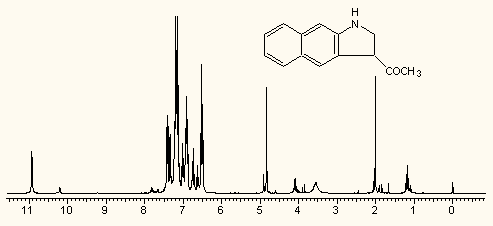
Different chemical and other adds
It is not easy for the reviewer to decide what and how to include, because these extras are changing quickly and depending on the owned version (freeware or standard
vs. pro
vs. ultra versions etc.), sometimes add-ins can be installed only into the purchased version, sometimes they are free, sometimes they are to be purchased separately or available as an Internet-based service (see
table 1. too). It is
highly recommended to look for information at the home pages of the manufacturers before making any decisions.
Molecular weight, and constitution %:
All of the programs.
NMR:
1H and 13C: KnowItAll, ChemBioDraw (with ChemNMR module in the Ultra version), ChemDoodle, ChemSketch (to be purchased), Accelrys Draw (with ACD/Labs Add-ins, only for versions 3.x), MarvinSketch (NMR Prediction, requires licence, free for academics)
19F, 31P and 15N NMR: ChemSketch (to be purchased)
The chemical shifts are not ab initio calculated, but determined with the aid of the applied databases; therefore, the precision of the results is a function of the included molecules. The 13C NMR module of the DrawIt/KnowItAll (if the full suite is installed) and ACD/CNMR can show molecules possessing analogue chemical environment similar to those of the carbons in question. ChemSketch has also developed this scheme into their NMR prediction modules through their "calculation protocol" environments.
MS Fragmentation tools:

The MS fragmentation tool mimics the molecular fragmentation in a mass spectrometer and it allows the user to break existing structures across one or more bonds. ChemBioDraw, ChemSketch, ChemDoodle, DrawIt and Chemistry 4-D Draw have this tool; the first three applications have the most advanced one, which allows the user to fragment in zigzags and calculate the mass resulting from multiple fragment losses (if you need more, use ACD's MS Fragmenter or a similar package).
In ChemDoodle and ChemBioDraw this tool can be used to perform simple synthesis/ retrosynthesis-type fragmentations as well.
IR: KnowItAll, ChemSketch (with UV-IR Manager add-on):
Other chemical property prediction tools:
ChemBioDraw: logP, logS, CLogP, critical pressure, temperature and volume, Henry's constant, Gibbs energy, heat of formation, MP, BP, molrefraction. Some of these are available in the Ultra version only
ChemSketch: logP (free version). With ACD/PhysChem Suite: molar refractivity and volume, parachor, surface tension, index of refraction, density, polarizability, logP, logD, pKa, Hammett σ etc. For the time being, earlier direct interaction of I-Lab from within ChemSketch is not supported. Consult ACD-Labs home page for current details.
DrawIt/KnowItAll: logP, logD, pKa, Rule of Five (depending on licence).
Accelrys Draw: Rule of Five, isotopomer distribution, enumerate stereoisomers and Markush structures as well as ACD Lab's Physicochemical and NMR predictors (only for versions 3.x)
ChemDoodle: Many topological and constitutional indices, structural and analytical data, Rule of Five, H-bond donor/acceptor number, molar refractivity and volume, polar surface area, XlogP, many thermodynamic and physicochemical properties, isotopic distribution, etc.
MarvinSketch: protonation and pKa, logP and logD, charge and polarisability, generation of tautomers, resonances, conformation and molecular dynamics (using the Dreiding force field), 3D alignement, topology, surface area, Hückel analysis (HOMO, LUMO, localisation energy, etc.), H-bond donor/acceptor properties. The results of several of these calculations are shown graphically as isosurfaces as well. All of these are available in the basic/free version. You can try these calculator plugins online at http://www.chemaxon.com/marvin/help/calculations/calculator-plugins.html
These calculated properties are to be handled with critic.
ChemBasic Goodies of ChemSketch:
A bunch of handy utilities can be found in these extensions, among others handling of multipage documents, conversion to HTML, VRML or SDF formats, peptide, carbohydrate and nucleic acid builders.
Reaction interpretation and stochiometry tool of ChemSketch and ChemBioDraw 14
This is a handy utility for the synthetic chemists to calculate the molar quantities of reagents and products and the yields of the reaction (Accelrys Draw has a similar calculator as separate add-in):
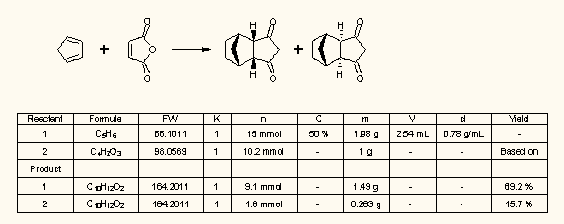
A handful of different unofficial add-ins are available for Accelrys Draw
13
Documentation
All major software applications were accompanied by ample printed documentations a decade ago. This good habit has disappeared by 2013. Nowadays the documentations are supplied in pdf format on the program DVD or in the downloadable package.
The available help system of the programs are good in the case of Accelrys Draw, ChemBioDraw, ChemSketch and DrawIt. The documentation found in the Chemistry 4-D is incomplete, several modules have no descriptions at all, no information could be found about the so-called Plasmid module even from the website of the program and it is dated from 2002. Friendly solution of Accelrys Draw that its Help contains a special section aimed at old ISIS/Draw users. The available pdf manual of ChemDoodle, unfortunately, lacks the index.
Worthy of note that for the sake of the user's convenient, the Help of the purchased version of ChemSketch contains a huge number of internet links to the instructions-for-author pages of chemical journals, moreover, and the majority of these instructions are available off-line in pdf format, too. Unfortunately, these latter are quite dusty and need update.
A very friendly attitude of ChemDoodle that in addition to the data, computed data, nomenclature, file formats, etc. all literature references used by the development team are available.
The Help found in the current MarvinSketch is obsolete and the search/find function is unusable.
Drawing programs and the Web
Nowadays there are more and more applications (or rather, browser plug-ins or Java applets), which can be used for entering chemistry directly into the browser, for example to search an on-line database. A Java applet version of ChemSketch is available at no cost from the ACD website (via the ACD/Structure Drawing Applet). This is a full featured Java-based structure drawing editor including templates and periodic table. The earlier I-Lab add-on to ChemSketch is currently not supported. The ChemBioDraw Plugin automatically turns the web browser into ChemBioDraw whenever a page containing an embedded ChemBioDraw document is encountered. DrawIt can use spectroscopic databases. However, we do not deal with these in detail this time. Several Java applet versions are found among the programs mentioned in the
What else out there section, too.
Web developers will find it useful that in addition to creating graphics and chemical files, ChemDoodle can also create dynamic web components with the aid of a specialized javascript library. Based on HTML 5 technologies, this tool provides a developer platform for creating interactive applications not only for browsers, but also for mobile devices such as the iPhone, iPad and Android devices. For more information visit ChemDoodle's website.
Optionally, you can save your documents as online (cloud) files from ChemDoodle (needs iChemLab account) and ChemBioDraw and AccelrysDraw (these need a Dropbox account) in the cloud. This is also the method for transferring your data between ChemDoodle desktop and ChemDoodle Mobile. Of course, all applications can save and open files using general cload storage services, such as Onedrive.
Latest versions of Accelrys Draw, ChemDoodle, ChemBioDraw, ChemSketch and MarvinSketch support the direct access of chemical databases to retrive structures and/or different data.
- Accelrys Draw: its Structure resolver can look up chemical and IUPAC names, registry numbers and keys from several web services.
- ChemDoodle: free access to PubChem, ChemSpider and ChemExper to look up and download structures based on names.
- ChemBioDraw: Apart from ChemACX (find strucutures from names) only paid database services are available. The ChemBioFinder Hotlink window (30 days free trial) retrives data from several databases (looking up data of structures) and has direct accesses to ChemSpider. Querying of SciFinder for those having a valid individual subscription (ChemBioDraw ultra serves as drawing client and then it launches SciFinder and redirects.)
- ChemSketch: free access to PubChem, Emolecules and ChemSpider (looking up data of structures).
- MarvinSketch: free access to ChemSpider, PubChem and Chemicalize (looking up the data of structures.)
Some other web-features, such as direct e-mailing of a sketch in pdf format or the RSS newsfeeds to receive announcements on the dedicated bottom toolbar of ChemSketch belong already to the "bells-and-whistles" category.
On browsing the different chemistry-related forums or lists, it turns out that many user faces the problem how to create an image from a chemical sketch to publish it on the web. It takes a few seconds for an advanced user, but problematic for the novice. Apart from DrawIt, all of the reviewed programs already support this. As it has already been mentioned, a rule of thumb that never use the jpg format for sketches, but the GIF or PNG formats instead! To know more about the GIF, PNG and JPG (JPEG) formats, read this
sidebar.
Summary
Apart from small corrections, no new features can already be added to the drawing functions of pure chemistry. Of course, little additions and corrections are continuously made, especially database functionalities are enhanced, such as the support for Markush structures, polymers, etc. are enhanced – this in mind the developers are recently concentrating on the intelligent to-and-fro chemical naming modules and the tools for viewing and representing spectra of different formats, and recently on the interactive connection with the internet.
For the time being, ChemBioDraw, ChemDoodle and ChemSketch are the most powerful chemical drawing applications. Old ChemWindow has been replaced by the DrawIt/ReportIt modules of the KnowItAll Analytical Systems package – no special novelties but excellent team-work with spectroscopy and chromatography. Similarly, the successor of ISIS/Draw can not show up any important novelties as its drawing features are concerned. Chemistry 4D Draw has not been developed for several years either.
Progress never stops, hardware keeps growing stronger and stronger, and the developers have to add something new every year, therefore the originally really drawing-only applications have evolved into a complex of
- full-featured editors of chemical, textual, spectroscopical and graphical objects for
- publication (classical or web-based) or
- e-notebook type data storage.
- several chemical tool sets:
- MS fragmentation tool, TLC plate tool, chemical table editor, different templates and alike;
- quick 2D → 3D conversion and visualisation for publication purposes;
- conversion of structures to names and vice versa, generation of stereo descriptors;
- Passive (one-way) database use:
- intelligent template-like use: quick look-up of natural or commercial products by their trivial, IUPAC names or registry numbers;
- Retrieval of data or manufacturers of compounds from the internet
- Prediction of spectroscopic data (NMR-shifts) based on databases.
- the input modules of other applications:
- active chemical databases, molecule and reaction retrieval, management of chromatographic and spectroscopical data, peak search, etc.;
- calculation modules (such as physical-chemical data, QSAR parameters etc.);
- available on-line services.
The comparison of the discussed packages is not an easy task, as in each one the potential user can find clear advantages compared to the others as well as weak points, too. The judgement of a program is highly dependent on the routine and knowledge of the user: better results can be achieved with a very well known and mastered, but less sophisticated program than with a partly known one full with bells-and-whistles. The user-friendliness is also relative as those who know well for example the different available keyboard shortcuts of an application can work more efficiently and quickly with this one than with another one. According to my opinion, if only plain chemical drawings are in question, all of the applications can be equally useful. In the case of more complicated drawings ChemSketch or ChemBioDraw is the most recommendable. ChemDoodle is also very good. When the management of spectra and chromatograms is a must, DrawIt or ChemSketch (the appropriate subsets of the suite) should be preferred. If 3D-modeling is also desired, you may consider ChemBioDraw + Chem3D. Choose ChemBioDraw when biochemical sketches are in question. Apart from ChemDoodle, these applications serve as the input modules of their appropriate chemical databases – another point of view for the proper judgement of these programs. Chemistry 4-D Draw contains only the minimum drawing features, it can be used as a name generator, too.
A power-user is usually not satisfied with one application only; some features are available in one program only, others are better in another one. File exchange via common formats is usually possible, and the higher-level mutual support of applications of even competitors (such as the ACD add-ins in the CambridgeSoft or Accelrys programs) is clearly beneficial for everybody.
My personal order of preference:
1-2-3 – All of these are perfect for the creation of average drawings of average publications.
Chem(Bio)Draw – get the appropriate version according to your demand. The most versatile of all applications. No free version, no free web services are available.
ChemDoodle – highly rising application. Much cheaper than Chem(Bio)Draw.
ChemSketch – get the free or commercial version according to your demand.
4 – Accelrys Draw (free academic version)
5 – Commercial versions of Accelrys Draw, DrawIt or Chemistry 4D Draw: only if you need it because of another member of the suite, doesn't worth purchasing solely because of drawing. As to me, I use mainly ChemBioDraw, however, in certain cases ChemDoodle and ChemSketch are also necessary at hand.
What else out there?
Beyond the above-discussed drawing software applications there are several other ones to be considered for general use or only for experimentation. They are freeware or shareware programs, some are highly sophisticated, some are simple ones, some are only in beta-phase. Others are excellent for web applications and/or are running only within a web page as applets. Here are a few of them (apart from MarvinSketch and PLT, no extensive tests have been done)
MarvinSketch 14.0 (Windows, Linux, Mac)
(ChemAxon Ltd)
As previously mentioned, MarvinSketch has not been added to the above six software applications, as for the time being, it is not meant for the creation of publication quality complex drawings and some necessary features are missing. However, it has several other tools and features that entitles it for special attention
Although it is only a basic 2D drawing software, it includes some features characteristic only of modelling softwares, for example coloring of the atoms by type or display in ball-and stick and CPK models It is possible to import 3D molfiles (Sybyl, PDB, XYZ, etc.), it has separated 2D and 3D clean-up modules. InChi, Smiles or Smarts strings are interpreted and cleaned up as well. MarvinSketch is available not only as stand-alone application (javabean), but as Java applet for developers as well and may serve as the graphical input module of on-line databases (therefore query bond and atom types, R-logic, Markush structures, groups, etc. are also supported). It contains several chemical property calculation and prediction tools as already described above. Unique to MarvinSketch that the source of the molecule in the work-space can be inspected and edited in several file formats in the source editor. The browsing of MDL database files is also possible. Partner applications: MarvinView for quick 2D → 3D conversion and display, and MarvinSpace for high performance 3D molecule and surface visualization. It is integrated into a variety of database systems via JChem Base. As already mentioned, it has a name generator for the computation of the IUPAC names of compounds. For more details visit its home page.
The images in MarvinSketch are optionally antialiased on the screen. Therefore MarvinSketch is also excellent for drawing molecules or reactions intended to export as images for web pages The sketches can be exported to JPG, BMP, PNG, SVG, PPM, EMF or PDF files.
URL:
http://www.chemaxon.com/products/marvin/marvinsketch/
WinPLT 7.1 (Windows)
(Hans J. Reich)
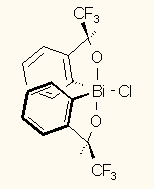
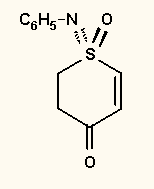
In the first part of the 90s the DOS based PLT was the masochists' drawing program with its dozens and dozens of keyboard shortcut combinations. It concentrates on drawing without the many bells-and-whistles of the above programs. Advanced users could absolutely master the drawing. For example, the height, breadth, slant, and rotation of text could be freely and continuously changed, as well as the fine adjustment of the bond crossings and many other parameters. The Windows version is already more user-friendly, but, unfortunately, some of the uniqueness of the original PLT had to be sacrificed. Even so, beyond the standard Windows fonts allowing only italic and bold formattings, a graphical font is available for free formatting. The technique and conception of drawing in PLT differs considerably from those in the above programs, which may seem odd at first (the program comes with ample help and tutorial). The presence of vector graphics can be strongly felt (the original PLT produced HPGL plotter files), but this allows many unusual solutions, such as direct embedding of keto or sulfone groups into rings or text labels etc, or creating special bonds by dismantling bonds into lines and reformatting them (such as the up/down double bonds in Figure 28) etc. It reads and writes only its own file format, can export to WMF, EMF, BMP, GIF, smoothed GIF and EPS graphical files or to the clipboard. Several other formats, e.g. MDL MOL or RXN files can be converted to PLT format by
Mol2Mol.
In addition to chemical drawing, in the Windows version modules for incorporating NMR spectra and producing X-Y graphs have been included. Some unique features: on-click addition of several common moieties, such as carbonyls, CH
2 groups with up/down bonds, etc; Bezier, sine, cosine, parabola, circle, Gaussian and Lorenzian curves are available; chemical slide-show (without Powerpoint) etc. Worth trying.
URL:
http://www.chem.wisc.edu/areas/reich/plt/winplt.htm
ICM-Chemist and
ICM-Chemist Pro (MolSofr)(Windows, Linux, Mac)
(Molsoft)
Drawing application and great suite of programs for 3D conversion, visualisation, pharmacophor searching, cheminformatics, etc. Worth to have a close look.
URL:
http://www.molsoft.com/icm-chemist.html
ChemPen and ChemPen3D (Windows)
(Hilton Evans)
A very useful shareware application, the advanced version supports NMR prediction, fast force field based geometry optimisation and 2D → 3D conversion, Hückel modeling, QSAR analysis. Worth trying.
URL:
http://chempen.software.informer.com/1.9/
JChemPaint 3.3 (Windows, Linux, Mac)
(JChemPaint Project)
Java based multiplatform, simple 2D drawing program. Stand-alone and java applet versions are available. Among others supports CML, SVG, Smiles and MDL MOL/SDF formats, and
13C NMR prediction.
URL:
http://jchempaint.github.io/
BKChem 0.14 (Windows, Linux, Mac)
(B. Kosata)
BKChem is a free chemical 2D drawing program written in Python, therefore it is a multiplatform application (Mac OS, GNU/Linux, Windows). It has basic drawing features. Supports CML, Adobe SVG graphics, PDF, Post Script, Open Office, etc. formats. Allows embedding good quality antialiased graphics into web pages (needs the Adobe SVG plugin.) No new version since 2010
URL:
http://bkchem.zirael.org
ChemicPen 2.6 (Windows)
(Cetramax)
Easy to use software for drawing 2D chemical formulae and reactions. Allows you to print and export formulae in other documents. No new version since 2010
URL:
http://www.cetramax.com/
ChemTool 1.6.14 (Linux)
(T.Volk - M. Kroeker)
Chemtool is a small program for drawing chemical structures on Linux and Unix systems using the GTK toolkit under X11.
URL:
http://ruby.chemie.uni-freiburg.de/~martin/chemtool/
ICEdit 2.3 (Windows)
(InfoChem)
ICEdit is a simple chemical structure and reaction editing tool. Supports the export and import of MDL's MOL, RXN and ISIS sketch files and Smiles strings. Java applet and JavaSript versions with query features is also available as a tool to create queries for searching structure and reaction databases.
URL:
http://infochem.de/products/software/icedit.shtml
SketchEI (Multiplatform)
(Sourceforge project, Dr. Alex M. Clark)
SketchEl is a Java-based interactive chemical molecule sketching tool, and a molecular spreadsheet data entry application. Accepts MDL's MOL and SDF formats, CML. Structures can be exported as SVG or PNG graphics.
URL:
http://sketchel.sourceforge.net/
 TouchMol
TouchMol (Windows, web and mobile appl.)
(Scilligence Corp.)
TouchMol is a suite of products offering a chemical/biological structure editor across a variety of platforms including web, mobile and touch devices, desktop and Microsoft Office applications. The Touchmol basic chemical drawing capabilities are enhanced with a simple peptide and DNA/RNA sequence editor and a few biological objects (gene, antibody). The tested web version has a few child diseases, such as lasso selection is not precise enough in documents with many objects. It is not very suitable for creating publication quality drawings (e.g. you cannot use sub/superscripts in atom labels or captions), however, useful for producing interactive lists or for web searches.
URL:
http://www.scilligence.com/web/touchmol.aspx
* * *
Applets only
Direct molecular structure input by using web browsers as interface is a very important branch of applications as web services have become of general use today. These are usually quite simple applets for generating basic (query) structures. Several of them import molecule files and the drawings can be exported to the usual document editors. Instead of going into details we refer here only to a recent overview
[8]. A few examples are here:
JME Editor 2012.06(Peter Ertl, Novartis Pharma AG)
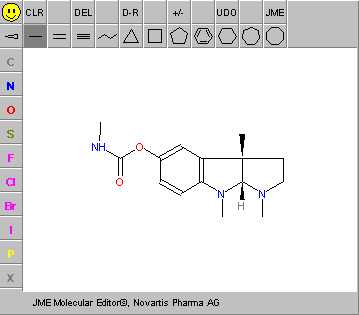
The simple and user friendly JME Molecular Editor is a free and often used Java applet which allows the user drawing, editing and displaying molecules and reactions directly within an HTML page. The editor can generate or input files in SMILES, MDL MOL/RXN or proprietary own formats. Its JME Professional version is not an applet but a stand-alone Swing application. Only pure molecules, query structures, and reactions can be drawn and saved/exported as molfiles. Creation of substructure queries is also supported. JME is an excellent tool for developers and may serve as the graphical input module for, e.g., on-line databases or property calculation services.
The applet may be used also in a depiction mode to visualise molecules:
URL:
http://www.molinspiration.com/jme
A new Javascript version of JME with several additional features is JSME by the same author. In addition, JSME supports molecule editing also on iPad, iPhone and Android touch devices.
URL:
http://peter-ertl.com/jsme/index.html
Another variant of the JME Editor is the Molinspiration
WebME Molecule Editor, based on Web2.0 Ajax technology.
URL:
http://www.molinspiration.com/docu/webme
 ChemWriter 3
ChemWriter 3 (Metamolecular LLC)
This is another simple javascript based multiplatform simple, but full-featured chemical structure editor and display applet for web and mobile applications, its main use is molecular structure input. Exports and imports MDL molfiles.
URL:
http://chemwriter.com/
ChemVector (Metamolecular LLC)
ChemVector is a javascript SDK library for web developers to display chemical structures in the browsers with high quality vector graphics. It renders structures directly from individual molfiles or CDX files on a server, or as inline content.
URL:
http://metamolecular.com/chemvector/
JSDraw 4 (Scilligence Corp)
A similar javascript-based multiplatform, cross-browser applet, a somewhat simpler variation of the above discussed TouchMol. Opens and saves ChemDraw, Marvin, MDL MOL/RXN/SDF files, Smiles format. Supports chemical spreadsheets, it has a built in sequence editor, SDF list viewer, etc.
URL:
http://www.scilligence.com/web/jsdraw.aspx

 Xemistry Web Sketcher 3.2
Xemistry Web Sketcher 3.2 (Xemistry GmbH)
A very advanced and useful multiplatform sketcher, a member of the Cactus toolkit for chemical information processing. As it is a server side application, it does not rely on plug-ins or applets or other components to be downloaded. It supports many formats to export and import chemical structures, among others most string formats (Smiles, InChi, SLN, Cactus, etc.), the most important molecule file formats (MDL MOL, PDB, MOL2, etc.) as well as the export of several graphical bitmap and vector formats (GIF, PNG, SVG, EPS, WMF, PDF, etc.). The sketcher program can easily be used in various web scenarios and linked to different services, etc.
URL:
http://www.xemistry.com
* * *
Add-ins
Chem4Word (University of Cambridge and Microsoft Corp.) (Windows)
Another interesting approach to include chemical information into documents is the chemistry add-in for Microsoft Word, a joint project of Microsoft Research and University of Cambridge. By creating inline "chemical zones" in Word, chemical information can be represented in a variety of ways: 2D chemical structures, names, chemical formulae or different data. In other words, instead of a static picture one can select with a few clicks what information should appear in the embedded fields. Chemical structures (called "zones") can be inserted from the web (PubChem or OPSIN) or from CML files. There is a very simple built-in structure editor in Chem4Word only enough for modification of imported structures. Thus, you need a more advanced drawing application capable of exporting 2D structures in CML format if you can not find and download a given structure. This project is only in experimental phase and can be used only with MS Word 2007 or 2010. No too much changes lately.
URL:
http://chem4word.codeplex.com and
http://research.microsoft.com/en-us/projects/chem4word/
TouchMol4Office (Scilligence Corp.)
TouchMol (optimized for touch devices) integrates molecular structure inputting and editing into the MS Office suite. (Word, Excel, PowerPoint and OneNote). It can be useful for creating interactive tables in Word or Excel, to display calculated properties, to import structures from SDF files and various chemical file formats, or web or Oracle/SQL servers. SAR analyser version is also available. Advanced users can tailor the documents with the aid of VBA. Chemical and biological structures can be drawn from within Microsoft PowerPoint.
URL:
http://www.scilligence.com/web/touchmol4office.aspx
top
References
1. Accelrys Draw 4.2 (Symyx Technologies, Inc) URL:
http://accelrys.com/products/collaborative-science/biovia-draw/
2. a) ChemBioDraw Ultra 14.0; PerkinElmer/CambridgeSoft Corporation, Cambridge, MA, USA; URL:
http://www.cambridgesoft.com/Ensemble_for_Chemistry/ChemBioDraw/Default.aspx
b) Review. URL: http://macinchem.org/reviews/chembiodraw14/chemdraw14_review.php (accessed 29.02.2016)
c) iPad version: URL: http://www.cambridgesoft.com/Ensemble_for_Chemistry/ChemDraw/ChemDrawforiPad/Default.aspx (accessed 29.02.2016)
d) iPad version review: URL: http://chemjobber.blogspot.hu/2013/06/product-review-chemdraw-for-ipad.html (accessed 29.02.2016)
3. DrawIt 9.0 (KnowItAll Academic Edition); Bio-Rad Laboratories Informatics Division, Philadelphia PA, USA; URL:
http://www.bio-rad.com/en-us/product/knowitall-academic-edition-free-chemistry-software
4. Testing and comparison was made on different laptops with Intel Core 2 or Core i7 CPU, Windows Vista SP2 and Windows 7/64 (International English releases). Text editor: MS 2010 (English). Printers: HP Laserjet 5, HP Deskjet 840, Epson XP-750.
5. a) ACD/ChemSketch 2015; Advanced Chemistry Development, Inc, Toronto, Canada; URL:
http://www.acdlabs.com
b) David Bradley: Chemical Structure Drawing Software, URL: http://www.sciencebase.com/aug04_iss.html (review, accessed 12.2.2016)
6. Chemistry 4-D Draw 8.4.6; ChemInnovation Software Inc., San Diego, USA; URL:
http://www.cheminnovation.com.
7. a) MarvinSketch 16.1.4, ChemAxon Ltd, Budapest, Hungary; URL:
http://www.chemaxon.com
b) A Review of Marvin. URL: http://macinchem.org/reviews/ChemAxon_review/marvin.php (accessed 12.2.2016)
8. a) P. Ertl: Molecular structure input on the web.
J. Cheminformatics,
2:1 (2010); URL:
http://www.jcheminf.com/content/2/1/1
b) A comparison of six chemical drawing packages. URL: http://homepage.mac.com/swain/Sites/Macinchem/Reviews/chem_drawing_packages/chem_draw_packages.html (accessed 29.01.2016)
9. a) ChemDoodle 8.0, iChemLabs LLC, Piscataway, NJ, USA; URL:
http://www.chemdoodle.com
b) ChemDoodle Review. URL: http://www.macinchem.org/reviews/chemdoodle7/chemdoodle7review.php
c) Exploring ChemDoodle Web Components. URL: http://www.macresearch.org/3d-chemdoodle-web-components (accessed 12.2.2016)
10. Other comparative reviews on chemical drawing programs and articles dealing with sketching:
a) A list of useful links to sites: URL: http://en.bio-soft.net/chemdraw.html (accessed 12.2.2016)>
b) Macs only, but useful in case of the Windows versions, too: URL: http://macinchem.org/reviews/ (accessed 12.2.2016)>
c) Vikas Anand: Comparative Evaluation of Freely Available Chemical Structure Drawing Softwares.
URL: http://www.pharmainfo.net/reviews/comparative-evaluation-freely-available-chemical-structure-drawing-softwares (accessed 17.9.2014)
d) A. M. Clark: Basic primitives for molecular diagram sketching. J. Cheminformatics, 2:8 (2010)
URL: http://www.jcheminf.com/content/2/1/8 (accessed 12.2.2016)>
11. An old and much shorter version of this document can be found at:
a) T. Gunda: Internet J. Chem. 3, L 25 (2000) (ISSN 1099-8292)
b) T. Gunda: Magyar Kémiai Folyóirat 104, 25-33 (1998)) (in Hungarian)
12. OSRA: Optical Structure Recognition Application. URL:
http://cactus.nci.nih.gov/osra (accessed 12.2.2016)
13. Accelrys Draw add-ins:
http://accelrys.com/products/collaborative-science/biovia-draw/add-ins.html (accessed 12.2.2016)
 top
top  Previous part
Previous part 
Download this article in Adobe pdf format StructureDrSw_2016_1.pdf (~3.0 MB)
|
Tamas E. Gunda ©
|

|
Maintained with the EditPad Pro  editor. Optimized for the Firefox editor. Optimized for the Firefox  browser browser
|
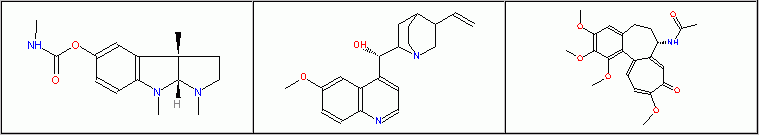
 The editing of structural text is the least convenient and limited in Accelrys Draw (the old, but more flexible ISIS/Draw mode can optionally be activated) and MarvinSketch (obviously only database applications have been taken into consideration). In certain cases, the method used in ChemDoodle is also complicated (e.g. the adding of Greek letters). If editing of a special atom label is difficult, the solution is simply to create a caption text and after positioning to group it with the parent structure.
The editing of structural text is the least convenient and limited in Accelrys Draw (the old, but more flexible ISIS/Draw mode can optionally be activated) and MarvinSketch (obviously only database applications have been taken into consideration). In certain cases, the method used in ChemDoodle is also complicated (e.g. the adding of Greek letters). If editing of a special atom label is difficult, the solution is simply to create a caption text and after positioning to group it with the parent structure.



 ChemBioDraw, ChemDodle and DrawIt allow the use of stacked labels (multiple lines).
ChemBioDraw, ChemDodle and DrawIt allow the use of stacked labels (multiple lines).

 In addition, all of the programs including MarvinSketch are able to assign the stereochemistry to all asymmetric tetrahedral atoms and double bonds according to Cahn-Ingold-Prelog (CIP) priority rules. All but ChemSketch do this on-the-fly while editing the structure. The method used in ChemSketch is quite clumsy: when editing the structure, you have to delete manully the previous R and S markers and ask for re-assignement of stereochemistry. (Direct internet search from the free version can partly compensate for this benefit.)
In addition, all of the programs including MarvinSketch are able to assign the stereochemistry to all asymmetric tetrahedral atoms and double bonds according to Cahn-Ingold-Prelog (CIP) priority rules. All but ChemSketch do this on-the-fly while editing the structure. The method used in ChemSketch is quite clumsy: when editing the structure, you have to delete manully the previous R and S markers and ask for re-assignement of stereochemistry. (Direct internet search from the free version can partly compensate for this benefit.)







 In the first part of the 90s the DOS based PLT was the masochists' drawing program with its dozens and dozens of keyboard shortcut combinations. It concentrates on drawing without the many bells-and-whistles of the above programs. Advanced users could absolutely master the drawing. For example, the height, breadth, slant, and rotation of text could be freely and continuously changed, as well as the fine adjustment of the bond crossings and many other parameters. The Windows version is already more user-friendly, but, unfortunately, some of the uniqueness of the original PLT had to be sacrificed. Even so, beyond the standard Windows fonts allowing only italic and bold formattings, a graphical font is available for free formatting. The technique and conception of drawing in PLT differs considerably from those in the above programs, which may seem odd at first (the program comes with ample help and tutorial). The presence of vector graphics can be strongly felt (the original PLT produced HPGL plotter files), but this allows many unusual solutions, such as direct embedding of keto or sulfone groups into rings or text labels etc, or creating special bonds by dismantling bonds into lines and reformatting them (such as the up/down double bonds in Figure 28) etc. It reads and writes only its own file format, can export to WMF, EMF, BMP, GIF, smoothed GIF and EPS graphical files or to the clipboard. Several other formats, e.g. MDL MOL or RXN files can be converted to PLT format by
In the first part of the 90s the DOS based PLT was the masochists' drawing program with its dozens and dozens of keyboard shortcut combinations. It concentrates on drawing without the many bells-and-whistles of the above programs. Advanced users could absolutely master the drawing. For example, the height, breadth, slant, and rotation of text could be freely and continuously changed, as well as the fine adjustment of the bond crossings and many other parameters. The Windows version is already more user-friendly, but, unfortunately, some of the uniqueness of the original PLT had to be sacrificed. Even so, beyond the standard Windows fonts allowing only italic and bold formattings, a graphical font is available for free formatting. The technique and conception of drawing in PLT differs considerably from those in the above programs, which may seem odd at first (the program comes with ample help and tutorial). The presence of vector graphics can be strongly felt (the original PLT produced HPGL plotter files), but this allows many unusual solutions, such as direct embedding of keto or sulfone groups into rings or text labels etc, or creating special bonds by dismantling bonds into lines and reformatting them (such as the up/down double bonds in Figure 28) etc. It reads and writes only its own file format, can export to WMF, EMF, BMP, GIF, smoothed GIF and EPS graphical files or to the clipboard. Several other formats, e.g. MDL MOL or RXN files can be converted to PLT format by 





